Many patients are unable to control their asthma with current treatments, and may prefer non-corticosteroid medications. Inhaled corticosteroids, although generally safe, have been associated with growth retardation in children and lower bone density in adults1. “There is an urgent need for alternative oral therapies to control symptoms and improve patient quality of life,” said Calman Prussin, immunologist and Vice President of Clinical and Translational Medicine at Knopp Biosciences, a privately-held drug discovery and development company based in Pittsburgh.
Dexpramipexole, an investigational orally-bioavailable synthetic amino-benzothiazole, was first developed as a treatment for amyotrophic lateral sclerosis (ALS) by Knopp Biosciences. Although it showed a significant benefit on a combined measure of function and survival in a phase 2 study, it failed to meet its primary end point in a large phase 3 trial2,3.
In the course of the ALS development program, however, dexpramipexole was observed to produce a significant and targeted depletion of eosinophils in the blood of ALS patients4.
Eosinophils and disease
Eosinophils are pro-inflammatory white blood cells thought to protect against parasitic infections, but in excessive numbers they can cause tissue and organ damage, as observed in eosinophilic gastroenteritis, atopic dermatitis, and in a heterogeneous group of rare disorders known as hypereosinophilic syndrome (HES). Most patients with HES are treated with high doses of corticosteroids, but many discontinue them owing to side effects, such as accelerated cardiovascular disease, osteoporosis and cataracts, and/or lack of efficacy.
Interestingly, around 50% of patients with asthma also have a high number of circulating eosinophils. Patients with eosinophilic asthma tend to have more severe disease, and more frequent asthma exacerbations.
In the past five years, three monoclonal antibody therapies that target interleukin-5 or its receptor have been approved by the US Food and Drug Administration and European Medicines Agency for the treatment of severe eosinophilic asthma. These biologics lower eosinophil numbers in both blood and tissue, with the weight of evidence indicating that eosinophil depletion reduces asthma exacerbations5,6.
“The approval of these biologics encouraged us to evaluate dexpramipexole in open-label pilot trials in patients with eosinophil-associated diseases,” said Michael Bozik, CEO of Knopp Biosciences.
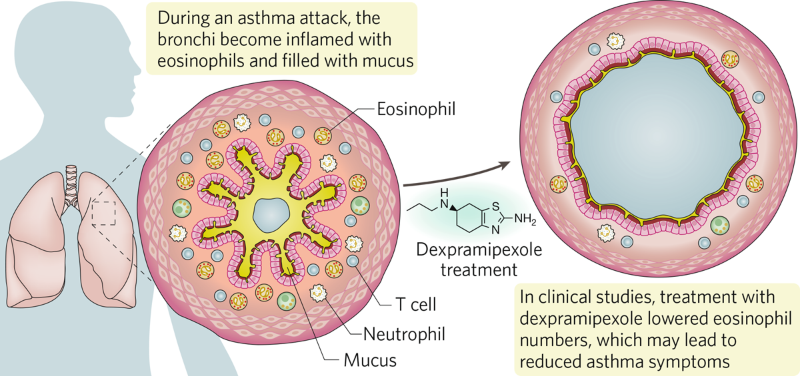
In collaboration with researchers at the National Institutes of Health, Prussin and colleagues at Knopp Biosciences showed in a phase 2 study that 12 weeks of dexpramipexole led to eosinophil depletion in the blood and in inflamed tissues of 10 patients with HES7. 40% of subjects were able to lower the prednisone dose required to control symptoms by ≥50%, the study’s primary endpoint. Notably, in 3 of the 10 patients, dexpramipexole lowered eosinophil counts to zero after 3 months on the drug, and over the next 2–4 months these 3 patients were able to taper off all oral corticosteroid medication, while maintaining control of their HES symptoms. Dexpramipexole was well tolerated, underscoring the potential of the drug as a steroid alternative for HES (Fig. 1).
Fig. 1 | Treatment with dexpramipexole in severe asthma attacks. Eosinophils are reduced, clearing the airway.
Phase 2 studies in eosinophilic asthma
Currently, Knopp Biosciences is sponsoring a randomized, placebo-controlled phase 2 trial of dexpramipexole in patients with poorly-controlled, moderate-to-severe eosinophilic asthma. This investigational trial will evaluate the effects of three dose levels of oral dexpramipexole for 12 weeks on peripheral blood eosinophil count, lung function, and asthma control. Additionally, the study will seek to determine the minimum effective dose needed for eosinophil lowering in both the blood and respiratory tract, which is an important step before studying dexpramipexole in phase 3 trials. The results are expected in 2021.
Knopp Biosciences is also collaborating with a consortium of UK asthma investigators in carrying out a phase 2 trial of dexpramipexole in patients with severe eosinophilic asthma. “This study seeks to assess if dexpramipexole is effective at decreasing severe asthma exacerbations,” explained Prussin. The results of this trial are expected by the end of 2023.
Evidence to date indicates that dexpramipexole depletes eosinophils by inhibiting the maturation of eosinophils in the bone marrow. “Because dexpramipexole has no direct effect on mature eosinophils, it takes about one month of treatment to see a robust reduction in circulating eosinophils,” explained Bozik. “We look forward to working with partners to study its tolerability and examine its clinical benefits in larger phase 3 patient populations,” he added.
The fact that dexpramipexole is orally administered makes it an attractive potential treatment alternative for alleviating eosinophil-induced inflammation. “If confirmed in phase 3 trials, the ability of dexpramipexole to lower eosinophils could transform the lives of patients with eosinophil-associated diseases,” Bozik concluded.
References
-
Sutter, S.A. & Stein, E.M. Curr. Osteoporos Rep. 14, 106–113 (2016).
PubMed Article Google Scholar -
Cudkowicz, M. et al. Nat. Med. 17, 1652–1656 (2011).
PubMed Article Google Scholar -
Cudkowicz, M.E. et al. Lancet Neurol. 12, 1059–1067 (2013).
PubMed Article Google Scholar - Dworetzky, S.I. et al. Blood Cell. Mol. Dis 63, 62–65 (2017).
- Legrand, F. & Klion, A.D. J. Allergy Clin. Immunol. Pract. 3(2), 167–174 (2015).
- Pelaia, C. et al. Front. Physiol. 10, 1514 (2019).
- Panch, S.R. et al. Blood 132(5), 501–509 (2018).

Comments are closed.